AI explains unknown mechanisms of Diazepam (Valium)

Behind the Pill, Issue #2
Abstract
Most drugs on the market produce side effects that can vary greatly in both severity and frequency. Often, the precise mechanisms driving these adverse effects are poorly understood, making it difficult to anticipate them accurately. Numerous drugs have been withdrawn due to unforeseen side effects, often after causing harm to many patients. A notable example is the anti-inflammatory drug rofecoxib (Vioxx), which was removed from the market in 2004 after being linked to an increased risk of heart attacks and strokes and after affecting dozens of thousands of patients.
To accelerate the understanding of existing drugs, and their repurposing for new therapeutic applications, Kantify used Artificial Intelligence (AI) through its AI drug discovery platform, Sapian. This effort involved evaluating the potential mechanisms of action for over 78,000 repurposable or natural drugs, resulting in more than 1.4 billion screened interactions—making it one of the most extensive AI-driven drug screenings to date. This novel approach not only allows Kantify to identify new uses for established drugs but also to predict and elucidate their potential side effects.
In this second installment of the "Behind the Pill" series, Kantify focuses on Diazepam, initially commercialized as Valium. As a prominent member of the benzodiazepine family, alongside Xanax, Diazepam has been used for over half a century to relieve acute anxiety. However, its widespread use carries notable risks, including drowsiness, fatigue, muscle weakness, and long-term dependence. When used during pregnancy, Diazepam has been linked to an increased risk of congenital malformations in newborns, such as cleft lip and palate, congenital heart defects, and neurodevelopmental impairments.
In this study, Sapian identified over 442 proteins potentially interacting with Diazepam, helping to explain its wide-ranging biological effects. Notably, the AI identified protein groups involved in the development of the roof of the mouth, heart, and brain, potentially elucidating the drug's harmful effects on fetal development. Additionally, Sapian predicted a possible impact of Diazepam on male fertility—an effect not previously listed in clinical descriptions and worthy of further investigation.
Through Diazepam’s example, Kantify explains how AI can be a powerful tool for drug developers and regulators. With AI, they can better anticipate drug side effects much before they reach the market, perform more informed drug design, and therefore decrease the pitfalls and costs of drug development and commercialization.
A peak Behind the Pill
At Kantify, we recently announced the capabilities of our AI technology Sapian to predict and explain the wanted and unwanted actions of well-known drugs. After analyzing the possible repurposing routes of Sildenafil (Viagra™), we now turn our attention to another iconic medication: Diazepam, also known as Valium™. As one of the most prominent members of the benzodiazepine family next to Xanax™ and Klonopin™, Valium has a long history of medical use, marked by its extensive prescription for a variety of conditions.
Figure 1: two boxes of Diazepam (left) and its molecular structure (right).
Valium became widely known in the 1960s and 1970s, at one point being the most prescribed drug in the United States, owing to aggressive marketing and its effectiveness in managing anxiety disorders, muscle spasms, and seizures. Its ability to provide rapid relief made it a popular choice for physicians. However, with newer alternatives and increased awareness of the risks of side effects and dependence, its use has shifted. Today, Diazepam is primarily prescribed for acute anxiety, alcohol withdrawal, seizure management, muscle pain and as a sedative before procedures1, but is typically limited to short-term use to mitigate long-term side effects.
Diazepam’s most common side effects, such as drowsiness, dizziness, muscle weakness and short-term memory issues directly stem from its primary actions1. Long-term use of Diazepam can also lead to physical and psychological dependence, with withdrawal symptoms ranging from anxiety and tremors to potentially life-threatening seizures if the drug is stopped suddenly. As Diazepam freely crosses the blood placenta barrier1, those effects are also frequently observed in newborns of mothers under treatment. Consequently, some infants are born with the "floppy infant syndrome", characterized by poor muscle tone, which can lead to feeding difficulties and other complications1.
Additionally, there are suspicions that Diazepam intake during the first trimester of pregnancy increases the chances of miscarriage and congenital malformations, especially when taken at high doses. The most commonly suspected malformations include cleft lip and palate, congenital heart defects, and broader neurological impairments which can cause changes in brain chemistry and behavioral abnormalities1, 2.
The complexity around forecasting and understanding a drug’s side effects
Navigating the side effects of any drug is a complex endeavor, often arising from a medication’s ability to affect unintended biological pathways. Drugs are designed to target specific systems or processes in the body, but the intricate interplay between different systems means that their effects rarely remain isolated. A medication that influences neurotransmitter activity, for example, may also affect cardiovascular or respiratory function, even if those areas weren’t the intended target.
The case of Diazepam is even more complex. Diazepam is metabolized primarily in the liver through the cytochrome P450 enzymes, particularly CYP2C19 and CYP3A4. It undergoes biotransformation into several active metabolites, including desmethyldiazepam, temazepam, and oxazepam, all of which contribute to its prolonged effects (fig 2).
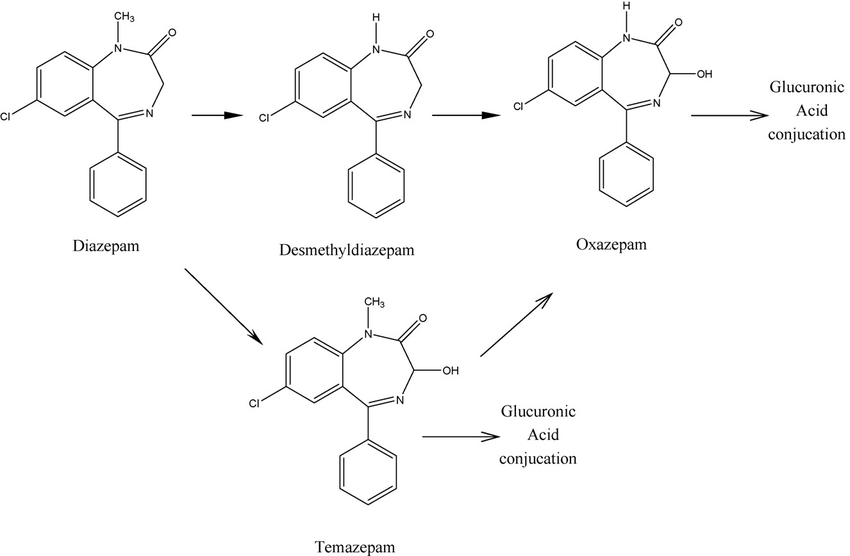
Figure 2 - An overview of Diazepam’s metabolic pathway, courtesy of Rouini et al.3
While there is a strong consensus today regarding the causes of both the beneficial and unwanted side effects of Diazepam, which is through its amplification of the activity of gamma-aminobutyric acid (GABA), a number of side effects observed through real world evidence cannot be explained through this mechanism of action alone. As such, there is an important need to further clarify how Diazepam induces these effects, and inform doctors and patients of possible risks of this drug.
One way Diazepam could induce these effects is through a polypharmacological profile - meaning that the drug and its metabolites interact with more than a single target. While it is now commonly understood that most small molecule drugs interact with multiple proteins, it is still considered difficult to identify exactly which ones, as the human body contains approximately 18,000 different types of proteins.
Using AI to identify drug interactions and potential risks
In recent years, computational methods have become essential in managing this complexity. Predictive models and machine learning are increasingly used to simulate drug interactions across these vast protein networks, allowing scientists to better anticipate both beneficial and harmful outcomes before clinical trials. While these tools can be effective to predict the interactions between drug candidates and standard proteins (cytochromes, nuclear receptors, ion channels), they usually fail to generalize to the entire human proteome.
At Kantify, we are leveraging the power of our novel AI technology , Sapian, to transform drug discovery by predicting complex interactions between small molecules and protein targets. After extensive training on hundreds of millions of interactions, Sapian can accurately predict how any small molecule interacts with all human proteins. In a virtual screening, we evaluated 78,000 repurposable and natural drugs - including all prescribed benzodiazepines -, generating more than 1.4 billion interactions. This large-scale effort represents one of the most comprehensive screenings to date, offering unparalleled insights into potential drug repurposing and protein targeting in the human proteome.
In practice, we use AI to move beyond statistical uncertainties by providing a list of targets likely to interact with a small molecule such as Diazepam which is the focus of the present article. This provides a practical map for researchers to test these interactions directly in lab settings, ultimately aiming to detect risks earlier than what is currently possible.
Capturing Diazepam’s unwanted effects
To visually capture the polypharmacological profile of Diazepam, we have generated a 2-dimension atlas of the human proteome as seen by Sapian (fig 2.a). Unlike a traditional geographical atlas, where the Y-axis represents the North to South prime meridian, and the X-axis represents the East to West equator, our atlas’ axes do not have a similar meaning. Instead, rather than absolute location, what matters in our map is proximity. More specifically, when proteins are close to one another, Sapian perceives them as being similar - in terms of structure, drugability, biological function, or other important features.
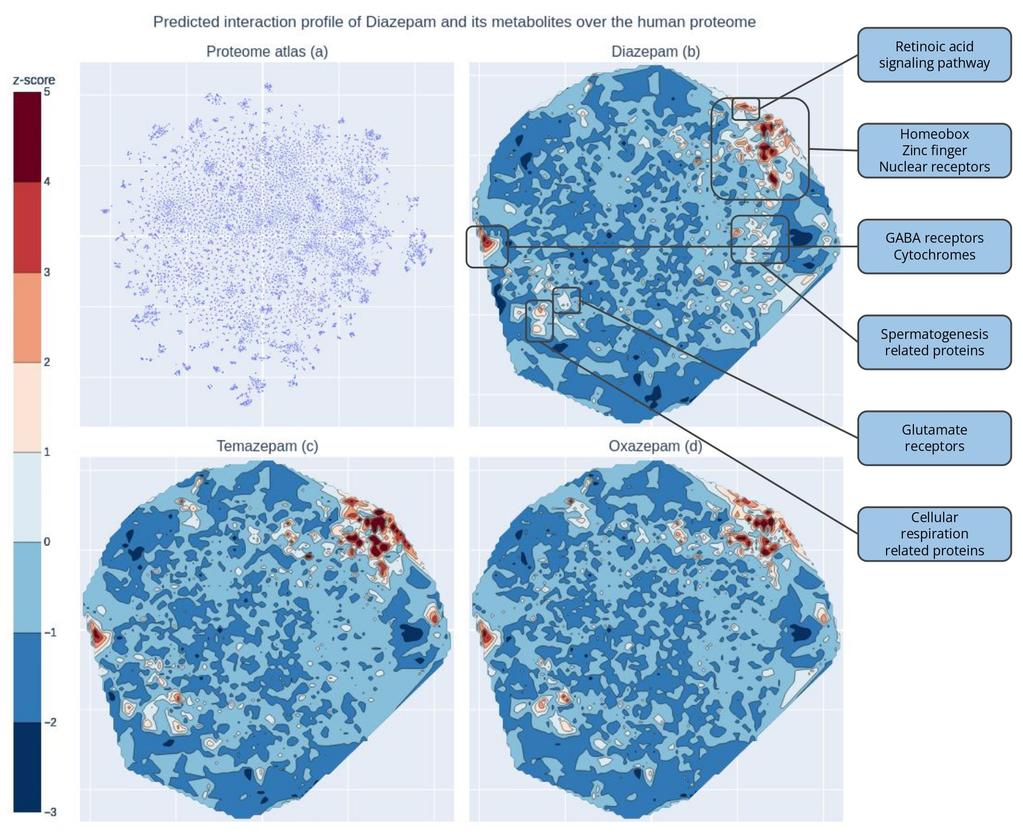
Figure 3 - (a) The learned representation of Sapian of the human proteome. Each dot represents one single protein in the human proteome. (b, c, d) Kantify’s Target Atlas for Diazepam, Temazepam and Oxazepam respectively. Warm zones indicate a higher likelihood that Diazepam interacts with proteins in these areas.
A colormap showcasing the predicted affinity of Diazepam and some of its metabolites across 18,000 proteins is overlaid on the atlas (fig 2.b, 2.c, 2.d). 442 novel proteins appear in the warm zones of the colormap which highlights high-affinity interactions. Interestingly, most of these proteins have little to no public data available, underscoring Sapian’s potential to uncover new biological insights and expand our understanding of unexplored protein interactions.
Upon a deeper exploration of the warm zones on the atlas, we identified several key protein interactions. As anticipated, GABA receptors and cytochromes involved in Diazepam's metabolism were prominent, confirming established knowledge. More intriguingly, glutamate receptors were also highlighted in Oxazepam’s top predictions. This is particularly compelling, given that glutamate and GABA pathways are known to interact closely4. Some effects linked to glutamate receptors, such as memory consolidation5, may explain Diazepam's side effects like short-term memory loss, further connecting these pathways to both its therapeutic and adverse effects. However, confirming that Diazepam exerts direct modulation on these receptors or if it influences them indirectly through downstream signaling cascades would require further research.
In the northeastern region of the atlas, a notably warm zone densely populated by transcription factors such as homeobox and zinc finger proteins can be observed. These proteins play critical roles during embryonic development and typically have low to no expression levels in adults. Their heightened presence in Diazepam's interaction profile may offer an explanation for the increased risk of congenital malformations reported in newborns when the drug is taken during the early stages of pregnancy. The disruption of these key developmental regulators could interfere with normal fetal growth, highlighting the potential risks of Diazepam during critical stages of gestation.
This same region of the atlas is also populated with important and well-studied nuclear receptors, including the estrogen, progesterone, and androgen receptors, as well as thyroid and mineralocorticoid receptors. These interactions are well documented, largely through the U.S. Tox21 screening program6, 7. Literature has been surprisingly silent about the potential consequences of these interactions. Notably, Diazepam is suspected of increasing the odds of spontaneous abortion8, 9, and recurrent spontaneous abortion has been linked to progesterone receptor dysregulation10, which makes these findings particularly significant for further research.
To go one step further, we utilized the Gene Ontology (GO) knowledge base11 to classify the top 442 predicted protein targets by their biological functions. This approach helped group proteins based on biological function, offering a clearer picture of the systems Diazepam potentially affects.
We also conducted an enrichment analysis to evaluate the statistical significance of each functional group identified. The fold enrichment value indicates how frequently a specific biological process appears in the group of 442 proteins compared to what would be expected in a random set. For instance, a fold enrichment of 2 means that this process is represented twice as often in the selected targets than in a random selection.
The largest groups identified by the analysis predominantly involved functions related to transcription and cell cycle regulation—an expected finding given the high representation of transcription factors among the 442 predicted protein targets. Within these largest groups, we identified five clusters that are particularly relevant to the reported side effects of Diazepam, all showing a high fold enrichment factor (Table 1).
The first cluster relates to the retinoic acid signaling pathway, which is crucial for regulating cell differentiation and organ formation during embryonic development. The next three clusters are linked to the development of the roof of the mouth, brain, and heart, respectively, while the fifth cluster is associated with spermatogenesis.
| Gene ontology ID | Biological process | Protein count | Fold enrichment | p-value |
|---|---|---|---|---|
| GO:0048384 | Retinoic acid signaling pathway | 10 | 28.02 | <0.001 |
| GO:0060021 | Root of mouth development | 14 | 8.90 | <0.001 |
| GO:0007420 | Brain development | 24 | 4.96 | <0.001 |
| GO:0007507 | Heart development | 20 | 3.46 | <0.001 |
| GO:0007283 | Spermatogenesis | 19 | 1.96 | 0.001 |
Table 1 - Results of the gene ontology analysis for 5 protein groups of interest.
Clearly, the biological processes in table 1 overlap nearly perfectly with the suspected side effects of Diazepam in embryos detailed in the introduction. This result is particularly striking for a variety of reasons. Firstly, over 12 000 biological processes exist in the GO knowledge base, meaning that the odds of Sapian randomly picking biological processes that match real world evidence is vanishingly small. Secondly, Sapian derives these insights purely through mechanistic predictions, by forecasting interactions between molecules and proteins, rather than relying on knowledge from literature, to which Sapian does not have access. Together, this highlights that Sapian can predict important health effects of drugs prior to human administration, which in turn can help to develop better, safer drugs.
In the following section, we will dive deeper into Diazepam’s potential impact on fetuses exposed to the drug, especially during the first trimester. While spermatogenesis emerged as a weaker finding, existing studies in animal models suggest a decline in fertility metrics under Diazepam use12, 13, 14. Although these studies have a number of limitations, they nonetheless offer some evidence to warrant further exploration when combined with our findings.
Examining Diazepam's impact on fetal development in early pregnancy
The first trimester of pregnancy is a critical period for embryonic development, where key processes such as organogenesis, neural tube formation, and limb development occur. During these initial 12 weeks, the foundations for all major organs and systems are established, making it a highly sensitive phase for any environmental influences, including medication.
Despite its suspected risks, Diazepam and other benzodiazepines continue to be prescribed during pregnancy. According to a global analysis, approximately 1.9% of pregnant women are prescribed benzodiazepines, with the highest prevalence in the third trimester (3.1%)15. Finding the best way to treat the mother's acute anxiety while minimizing the risks to the fetus is essential.
Today, the standard approach to assessing risks related to drug exposure involves traditional statistical analysis methods. While these are powerful, they come with significant drawbacks. They require extensive data to yield reliable results and are susceptible to various biases, such as sample size limitations, selection or recall biases. This often means that harmful effects are only identified after considerable damage has already occurred. Additionally, confounding factors like genetic variability or the specific medical indication for use can drastically alter study outcomes, contributing to the 50 year long debate about the teratogenic effects of diazepam.
In the following sections, we examine three categories of congenital conditions potentially linked to Diazepam exposure: orofacial clefts, congenital heart diseases, and neurodevelopmental disorders. Despite extensive research, there is still no consensus on these risks, and the current medical advice is to use benzodiazepines cautiously until more conclusive studies are conducted.
Orofacial clefts
Orofacial clefts, including cleft lip with or without cleft palate, are among the most common congenital malformations, occurring in approximately 1 in 700 live births worldwide. These conditions arise from incomplete fusion of facial tissues during early pregnancy, with risk factors ranging from genetics to environmental influences like maternal smoking or alcohol use. Although surgical correction is available, complications may persist.
Child with cleft lip and palate, courtesy if Wikipédia.
Earlier studies suggested that Diazepam and other benzodiazepines taken during the first trimester of pregnancy could increase the risk of oral clefts16, 17. These findings initially raised considerable alarm within the medical community. Since then, numerous studies have attempted to replicate these results, the most recent ones having reported no significant association, leading to a more conservative view of the risks2, 18.
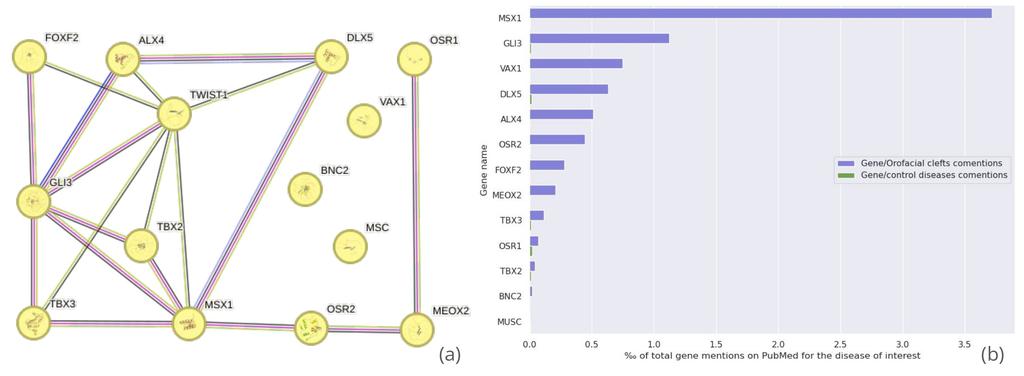
Figure 4 - (a) STRING-db network analysis of the 14 proteins associated with the "roof of mouth development" gene ontology group. Each line represents a known protein-protein interaction, with line thickness indicating the confidence level of the interaction. (b) Frequency of co-mentions in PubMed titles and abstracts between the 14 target proteins and "orofacial cleft" or related disease terms. Control bars are based on the average of 10 commonly studied diseases, such as hypertension and diabetes, for comparison purposes. Proteins mentioned less than 20 times on PubMed have been removed from the figure.
Proteins predicted to interact with Diazepam and associated with the "Roof of mouth development" gene ontology were selected for further analysis. StringDB was utilized to determine to which extent these proteins interact with each other (fig 4.a). Except for three isolated nodes, all of these proteins formed a well-connected network, suggesting a potential additive effect that might contribute to the observed risk of cleft lip and palate associated with Diazepam use. Additionally, the frequency of co-mentions of these proteins with terms like "orofacial cleft" and related synonyms in scientific literature was evaluated (fig 4.b). All of these proteins were significantly more frequently co-mentioned in association with orofacial clefts compared to other control diseases, reinforcing their possible relevance in this developmental condition.
A closer glance at the literature further supports these findings. For instance, studies indicate that loss of t-box transcription factor TBX3 (TBX3) function in the murine neural crest can lead to cleft palate formation in mice, highlighting the gene’s importance in craniofacial development19. Similarly, reduced homeobox protein MOX-2 (MEOX2) function has been associated with increased susceptibility to cleft palate20. Moreover, evidence points to possible links between mutations in ventral anterior homeobox 1 (VAX1)21, homeobox protein MSX-1 (MSX1)22, forkhead box protein F2 (FOXF2)23, and transcriptional activator GLI3 (GLI3)24 and the occurrence of orofacial clefts.
Neurodevelopmental disorders
There is growing concern that prenatal exposure to benzodiazepines could be linked to an increased risk of neurodevelopmental disorders, such as ADHD, autism spectrum disorders, intellectual disability, and communication and motor disorders. However, current evidence is limited, and recent systematic reviews have not been able to draw strong conclusions25.
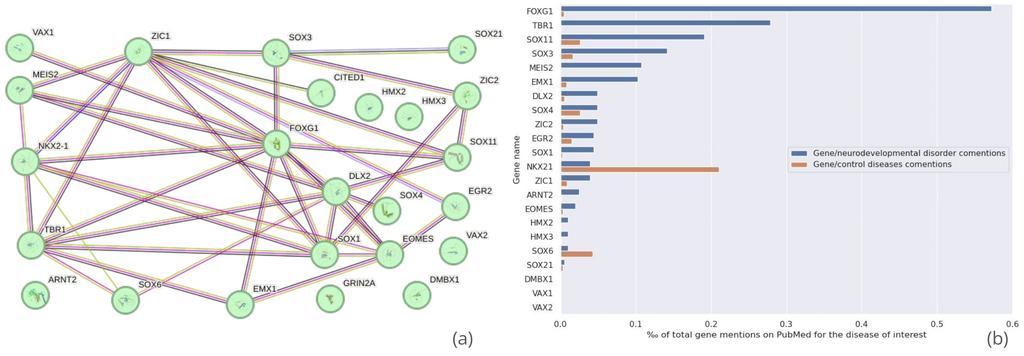
Figure 5 - (a) STRING-db network analysis of the 24 proteins associated with the "brain development" gene ontology group. Each line represents a known protein-protein interaction, with line thickness indicating the confidence level of the interaction. (b) Frequency of co-mentions in PubMed titles and abstracts between the 24 target proteins and "neurodevelopmental disorder" or related disease terms. Control bars are based on the average of 10 commonly studied diseases, such as hypertension and diabetes, for comparison purposes. Proteins mentioned less than 20 times on PubMed have been removed from the figure.
The StringDB analysis revealed that the proteins predicted to interact with Diazepam formed a highly interconnected network, suggesting substantial cross-talk. Some proteins, such as forkhead box protein G1 (FOXG1) and t-box brain protein 1 (TBR1), are linked to syndromes characterized by severe intellectual disability26, 27. Furthermore, transcription factor SOX-11 (SOX11) has been implicated in conditions such as microcephaly and floppy infant syndrome—disorders long suspected to be correlated with prenatal intake of Diazepam and other benzodiazepines28, 29. Homeobox protein Meis2 (MEIS2) appears to be a particularly promising gene for further exploration, as alterations in MEIS2 are believed to be associated with congenital heart malformations, intellectual disability, and facial dysmorphism, including orofacial clefts30.
Congenital heart disease
Congenital heart defects (CHDs) are among the most common birth defects, affecting approximately 1% of live births globally31. These structural anomalies of the heart can range from simple conditions, such as atrial septal defects (holes in the heart), to more complex issues like Tetralogy of Fallot, which affects blood flow through the heart.
While there is no clear consensus on the relationship between Diazepam and CHDs, experimental studies have raised concerns. A study demonstrated that Diazepam significantly reduced the beating activity in embryonic chick heart micromass cultures, suggesting potential interference with cardiac development32. Additionally, a cohort study in humans indicated a small increase in the risk of CHDs associated with prenatal exposure to Diazepam2.
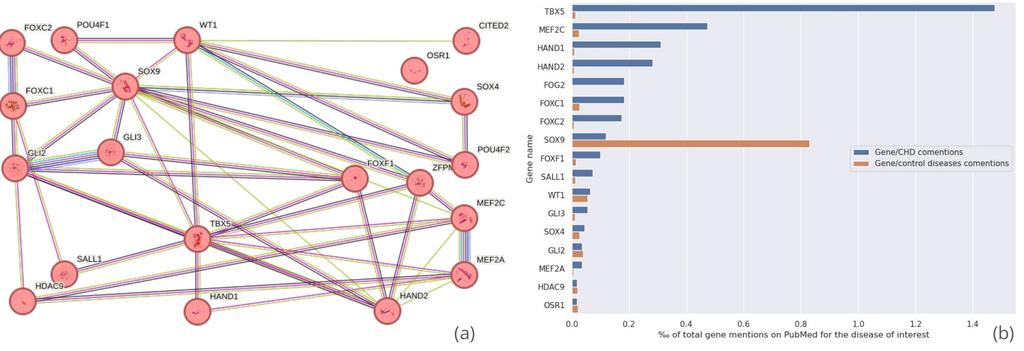
Figure 6 - (a) STRING-db network analysis of the 20 proteins associated with the "heart development" gene ontology group. Each line represents a known protein-protein interaction, with line thickness indicating the confidence level of the interaction. (b) Frequency of co-mentions in PubMed titles and abstracts between the 20 target proteins and "congenital heart disease" or related disease terms. Control bars are based on the average of 10 commonly studied diseases, such as hypertension and diabetes, for comparison purposes. Proteins mentioned less than 20 times on PubMed have been removed from the figure.
The analysis conducted in previous sections revealed several noteworthy findings related to genes involved in heart development. T-box transcription factor TBX5 (TBX5) emerged as a crucial gene for embryonic heart formation, with mutations linked to Holt-Oram syndrome—a disorder affecting both the heart and upper limbs, often resulting in atrial or ventricular septal defects33. Recent studies have also highlighted myocyte-specific enhancer factor 2C (MEF2C)’s significant role in the development of CHDs34. Additionally, members of the forkhead box (FOX) protein family were found to be associated with heart development and to be heavily implicated in the CHDs of humans35.
Another piece of the puzzle: retinoic acid signaling pathway
One of the strongest findings of the gene ontology clustering analysis is the involvement of the retinoic acid (RA) signaling pathway. Retinoic acid, a metabolite of vitamin A, plays a crucial role in regulating gene expression during embryonic development. It is essential for processes like cell differentiation, proliferation, and organ formation. The RA signaling pathway operates by binding to nuclear receptors, which in turn regulate the transcription of target genes crucial for the proper formation of structures like the heart, limbs, and craniofacial regions. During embryogenesis, precise RA levels are vital, as both excess and deficiency can lead to developmental abnormalities, including congenital malformations like cleft palate36.
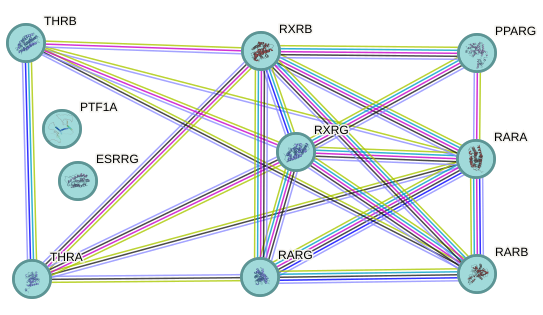
Figure 7 - STRING-db network analysis of the 10 proteins associated with the "retinoic acid signaling pathway" gene ontology group. Each line represents a known protein-protein interaction, with line thickness indicating the confidence level of the interaction.
A recent overview of the key functions of RA signaling during development has underscored its significant roles in heart anteroposterior patterning and forebrain development, lending support to our findings on the potential impact of Diazepam on embryonic development37. Notably, the study also emphasizes the critical role of RA in spermatogenesis, presenting an additional compelling reason to further explore the potential effects of Diazepam on male fertility.
Toward better preclinical identification of side effects
Our findings advocate for a broader perspective on drug side effects, one that moves beyond the traditional single-target paradigm to embrace the inherent complexity of polypharmacology of small molecule drugs. Although Diazepam has been used for over half a century, some of its side effects remain uncertain due to the lack of sufficiently robust studies that can definitively confirm or refute a correlation between exposure and adverse outcomes.
Computational methods like Sapian offer a new approach by enabling preclinical side effect identification by narrowing down the list of side effect inducing targets for testing beyond typical ones. At Kantify, our goal is not just to predict interactions but to uncover the hidden narratives within every molecule. Our journey with Valium and Viagra marks the beginning of a deeper exploration. We invite clinicians, biologists, and the pharmaceutical community to join us in challenging existing knowledge and discovering both the promising and problematic facets of drugs we thought we fully understood—for the benefit of patients everywhere.
Learn more
For inquiries or suggestions on which drug to explore next, reach out to us at Kantify. Curious about the off-targets of a small molecule that is in clinical trials or on the market? Get in touch, or follow us online to receive our next target Behind the Pill article.
Addendum
Below is the list of predicted targets that have been mentioned in the article, ordered by descending strength of signal. For access to the full list of 442 off-targets, please contact us
MCR GCR GLI3 EOMES GLI2 PRGR ANDR ZIC2 BNC2 DLX2 OSR2 SOX6 ESR2 VAX1 MEOX2 SALL1 ESR1 WT1 HAND2 TBX2 DMBX1 HMX3 PPARG FOXF2 RARG OSR1 SOX1 PTF1A FOXC1 RXRB HAND1 MEIS2 EGR2 FOXF1 ALX4 RXRG EMX1 TBX3 VAX2 MSX1 TBX5 SOX21 RARA SOX4 RARB DLX5 ZIC1 HDAC9 FOXC2 FOXG1 MEF2C SOX9 ARNT2 HMX2 MEF2A SOX3
Disclaimer
The information provided in this article is for informational purposes only and is not intended as medical advice. This content should not be used to diagnose, treat, cure, or prevent any medical condition. Always consult a qualified healthcare provider for advice regarding any medical concerns or before starting or stopping any medication. The insights and findings discussed are based on research and are not a substitute for professional medical guidance.
Authors
Jack Dawe, Nicolas Maignan, David Papazian, Maxime Georges, Caio Hudson de Souza, Rubal Ravinder, Ségolène Martin, Nik Subramanian
About Valium
"Valium® (diazepam) is a medication originally developed and marketed by F. Hoffmann-La Roche AG. Valium® is a registered trademark of F. Hoffmann-La Roche AG."
Sources
[1] DailyMed - DIAZEPAM- diazepam oral solution DIAZEPAM (diazepam oral solution- concentrate solution. (n.d.-b). Link to website
[2] Noh, Y., Lee, H., Choi, A., Kwon, J. S., Choe, S. A., Chae, J., Kim, D. S., & Shin, J. Y. (2022). First-trimester exposure to benzodiazepines and risk of congenital malformations in offspring: A population-based cohort study in South Korea. PLoS medicine, 19(3), e1003945. Link to journal
[3] Rouini, M. R., Ardakani, Y. H., Moghaddam, K. A., & Solatani, F. (2008). An improved HPLC method for rapid quantitation of diazepam and its major metabolites in human plasma. Talanta, 75(3), 671–676. Link to journal
[4] Wen, Y., Dong, Z., Liu, J. et al. Glutamate and GABAA receptor crosstalk mediates homeostatic regulation of neuronal excitation in the mammalian brain. Sig Transduct Target Ther 7, 340 (2022). Link to journal
[5] Riedel, G., Platt, B., & Micheau, J. (2003). Glutamate receptor function in learning and memory. Behavioural brain research, 140(1-2), 1–47. Link to journal
[6] Tox21. (2024, July 18). National Toxicology Program. Link to website
[7] PubChem. (n.d.). Tox21 - PubChem data source. PubChem. Link to website
[8] Grigoriadis, S., Graves, L., Peer, M., Mamisashvili, L., Ruthirakuhan, M., Chan, P., Hennawy, M., Parikh, S., Vigod, S. N., Dennis, C. L., Steiner, M., Brown, C., Cheung, A., Dawson, H., Rector, N., Guenette, M., & Richter, M. (2020). Pregnancy and Delivery Outcomes Following Benzodiazepine Exposure: A Systematic Review and Meta-analysis. Canadian journal of psychiatry. Revue canadienne de psychiatrie, 65(12), 821–834. Link to journal
[9] Sheehy, O., Zhao, J. P., & Bérard, A. (2019). Association Between Incident Exposure to Benzodiazepines in Early Pregnancy and Risk of Spontaneous Abortion. JAMA psychiatry, 76(9), 948–957. Link to journal
[10] Rahnama, R., Rafiee, M., Fouladi, S., Akbari-Fakhrabadi, M., Mehrabian, F., & Rezaei, A. (2019). Gene expression analysis of membrane progesterone receptors in women with recurrent spontaneous abortion: a case control study. BMC research notes, 12(1), 790. Link to journal
[11] Gene Ontology Resource. (n.d.). Gene Ontology Resource. Link to website
[12] Taher, M.A.; Anber, Z.N.H. Effect of diazepam on the reproductive system in male rats. World J. Pharm. Pharm. Sci. 2015, 4, 60–78. Link to journal
[13] Baumgartner, A., Schmid, T. E., Schuetz, C. G., & Adler, I. D. (2001). Detection of aneuploidy in rodent and human sperm by multicolor FISH after chronic exposure to diazepam. Mutation research, 490(1), 11–19. Link to journal
[14] Fogliano, C., Carotenuto, R., Cirino, P., Panzuto, R., Ciaravolo, M., Simoniello, P., Sgariglia, I., Motta, C. M., & Avallone, B. (2024). Benzodiazepine Interference with Fertility and Embryo Development: A Preliminary Survey in the Sea Urchin Paracentrotus lividus. International journal of molecular sciences, 25(4), 1969. Link to journal
[15] Bais, B., Molenaar, N. M., Bijma, H. H., Hoogendijk, W. J. G., Mulder, C. L., Luik, A. I., Lambregtse-van den Berg, M. P., & Kamperman, A. M. (2020). Prevalence of benzodiazepines and benzodiazepine-related drugs exposure before, during and after pregnancy: A systematic review and meta-analysis. Journal of affective disorders, 269, 18–27. Link to journal
[16] Safra, M. J., & Oakley, G. P., Jr (1975). Association between cleft lip with or without cleft palate and prenatal exposure to diazepam. Lancet (London, England), 2(7933), 478–480. Link to journal
[17] Saxén I. (1975). Associations between oral clefts and drugs taken during pregnancy. International journal of epidemiology, 4(1), 37–44. Link to journal
[18] Marinucci, L., Balloni, S., Carinci, F., Locci, P., Pezzetti, F., & Bodo, M. (2011). Diazepam effects on non-syndromic cleft lip with or without palate: epidemiological studies, clinical findings, genes and extracellular matrix. Expert opinion on drug safety, 10(1), 23–33. Link to journal
[19] López, S. H., Avetisyan, M., Wright, C. M., Mesbah, K., Kelly, R. G., Moon, A. M., & Heuckeroth, R. O. (2018). Loss of Tbx3 in murine neural crest reduces enteric glia and causes cleft palate, but does not influence heart development or bowel transit. Developmental biology, 444 Suppl 1(Suppl 1), S337–S351. Link to journal
[20] Jin, J. Z., & Ding, J. (2006). Analysis of Meox-2 mutant mice reveals a novel postfusion-based cleft palate. Developmental dynamics : an official publication of the American Association of Anatomists, 235(2), 539–546. Link to journal
[21] Zhang, B. H., Shi, J. Y., Lin, Y. S., Shi, B., & Jia, Z. L. (2018). VAX1 gene associated non-syndromic cleft lip with or without palate in Western Han Chinese. Archives of oral biology, 95, 40–43. Link to journal
[22] Salahshourifar, I., Halim, A. S., Wan Sulaiman, W. A., & Zilfalil, B. A. (2011). Contribution of MSX1 variants to the risk of non-syndromic cleft lip and palate in a Malay population. Journal of human genetics, 56(11), 755–758. Link to journal
[23] Bu, L., Chen, Q., Wang, H., Zhang, T., Hetmanski, J. B., Schwender, H., Parker, M., Chou, Y. H., Yeow, V., Chong, S. S., Zhang, B., Jabs, E. W., Scott, A. F., & Beaty, T. H. (2015). Novel evidence of association with nonsyndromic cleft lip with or without cleft palate was shown for single nucleotide polymorphisms in FOXF2 gene in an Asian population. Birth defects research. Part A, Clinical and molecular teratology, 103(10), 857–862. Link to journal
[24] Wang, Y., Sun, Y., Huang, Y., Pan, Y., Shi, B., Ma, J., Ma, L., Lan, F., Zhou, Y., Shi, J., Zhu, J., Jiang, H., Zhang, L., Xiao, X., Jiang, M., Yin, A., Yu, L., Wang, L., Cheng, J., & Yang, Y. (2017). The association study of nonsyndromic cleft lip with or without cleft palate identified risk variants of the GLI3 gene in a Chinese population. Journal of genetics, 96(4), 687–693. Link to journal
[25] Wang, X., Zhang, T., Ekheden, I., Chang, Z., Hellner, C., Jan Hasselström, Jayaram-Lindström, N., M D'Onofrio, B., Larsson, H., Mataix-Cols, D., & Sidorchuk, A. (2022). Prenatal exposure to benzodiazepines and Z-drugs in humans and risk of adverse neurodevelopmental outcomes in offspring: A systematic review. Neuroscience and biobehavioral reviews, 137, 104647. Link to journal
[26] FOXG1 syndrome: MedlinePlus Genetics. (n.d.). Link to website
[27] Rare Chromosome Disorder Support Group, & Nambot, S. (2020). TBR1–related disorder. In Rare Chromosome Disorder Support Group. Link to PDF file
[28] rarechromo.org. (2021). SOX11 syndrome. In rarechromo.org. Link to PDF file
[29] Hempel, A., Pagnamenta, A. T., Blyth, M., Mansour, S., McConnell, V., Kou, I., Ikegawa, S., Tsurusaki, Y., Matsumoto, N., Lo-Castro, A., Plessis, G., Albrecht, B., Battaglia, A., Taylor, J. C., Howard, M. F., Keays, D., Sohal, A. S., DDD Collaboration, Kühl, S. J., Kini, U., … McNeill, A. (2016). Deletions and de novo mutations of SOX11 are associated with a neurodevelopmental disorder with features of Coffin-Siris syndrome. Journal of medical genetics, 53(3), 152–162. Link to journal
[30] Giliberti, A., Currò, A., Papa, F. T., Frullanti, E., Ariani, F., Coriolani, G., Grosso, S., Renieri, A., & Mari, F. (2020). MEIS2 gene is responsible for intellectual disability, cardiac defects and a distinct facial phenotype. European journal of medical genetics, 63(1), 103627. Link to journal
[31] Wu, W., He, J., & Shao, X. (2020). Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990-2017. Medicine, 99(23), e20593. Link to journal
[32] Ahir, B. K., & Pratten, M. K. (2011). Association of anxiolytic drugs diazepam and lorazepam, and the antiepileptic valproate, with heart defects--effects on cardiomyocytes in micromass (MM) and embryonic stem cell culture. Reproductive toxicology (Elmsford, N.Y.), 31(1), 66–74. Link to journal
[33] Steimle, J. D., & Moskowitz, I. P. (2017). TBX5: A Key Regulator of Heart Development. Current topics in developmental biology, 122, 195–221. Link to journal
[34] Qiao, X. H., Wang, F., Zhang, X. L., Huang, R. T., Xue, S., Wang, J., Qiu, X. B., Liu, X. Y., & Yang, Y. Q. (2017). MEF2C loss-of-function mutation contributes to congenital heart defects. International journal of medical sciences, 14(11), 1143–1153. Link to journal
[35] Zhu H. (2016). Forkhead box transcription factors in embryonic heart development and congenital heart disease. Life sciences, 144, 194–201. Link to article
[36] Okano, J., Udagawa, J., & Shiota, K. (2014). Roles of retinoic acid signaling in normal and abnormal development of the palate and tongue. Congenital anomalies, 54(2), 69–76. Link to article
[37] Ghyselinck, N. B., & Duester, G. (2019). Retinoic acid signaling pathways. Development (Cambridge, England), 146(13), dev167502. Link to article